Home Grown
Sustainable Living
WWW.STEMCELL4UONLINE.COM CALL (833)278-3623



 INTRO TO STEM CELL CLICK ON VIDEOS:
INTRO TO STEM CELL CLICK ON VIDEOS:
WHAT DO THE SCIENTISTS SAY..
A new way to treat worn knee cartilage; surgery optional | Juana Mendenhall | TEDxPeachtree
Misconceptions On Stem Cells: Poh Tan at TEDxStanleyPark
Harnessing the potential of stem cells for new medicines: Diabetes
Doug Melton at TEDxBeaconStreet
YOUR STEM CELLS: FRIEND OR FOE?
Doug Frantz at TEDxSanAntonio 2013
STEM CELL THERAPY -- BEYOND THE HEADLINES:
Timothy Henry at TEDxGrandForks
Promises and Dangers of Stem Cell Therapies | Daniel Kota | TEDxBrookings

 IN memory of Dr. Wayne Gibbons,MD
IN memory of Dr. Wayne Gibbons,MD
 .
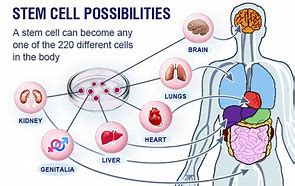
. 
WWW.STEMCELL4UONLINE.COM
CALL (833)278-3623
Medical Professional Viewing Only (Disclaimer)
This site was intended for education purposes only and strictly for use by medical professionals. The FDA recently re-confirmed, there is only one registered stem cell product, and while there is enormous promise in stem cell therapies, and thousands of ongoing experimental applications trying to establish efficacy, these are not at the point where they would meet the scientific standard.
The FDA has stated:
Stem cells, like other medical products that are intended to treat, cure or prevent disease, generally require FDA approval before they can be marketed. FDA has not approved any stem cell-based products for use, other than cord blood-derived hematopoietic progenitor cells (blood forming stem cells) for certain indications.
http://www.fda.gov/AboutFDA/Transparency/Basics/ucm194655.htm
This site is not intended for consumers.
If you are considering stem cell treatment in the U.S., ask your physician if the necessary FDA approval has been obtained or if you will be part of an FDA-regulated clinical study. This also applies if the stem cells are your own. Even if the cells are yours, there are safety risks, including risks introduced when the cells are manipulated after removal.
“There is a potential safety risk when you put cells in an area where they are not performing the same biological function as they were when in their original location in the body.” Cells in a different environment may multiply, form tumors, or may leave the site you put them in and migrate somewhere else.
If you are considering having stem cell treatment in another country, learn all you can about regulations covering the products in that country. Exercise caution before undergoing treatment with a stem cell-based product in a country that—unlike the U.S.—may not require clinical studies designed to demonstrate that the product is safe and effective. FDA does not regulate stem cell treatments used solely in countries other than the United States and typically has little information about foreign establishments or their stem cell products.
http://www.fda.gov/ForConsumers/ConsumerUpdates/ucm286155.htm
Stem cell therapies have enormous promise, but the science in each use is still in the developmental stage. Professional judgment and expertise is needed in using stem cells for any therapeutic use, and we urge anyone embarking on the use of stem cell therapies to consult the national health data bases to evaluate current information from clinical trials and the FDA websites on human tissue should also be consulted to get its current evaluation of any therapy.
 www.globalstemcelladvocates.com
www.globalstemcelladvocates.com
MODERN NEVER LOOKED SO GREEN.
Lorem ipsum dolor sit amet, mea ei populo nostrum consulatu, vis verear corpora ea. Ei nec alia iracundia vulputate, eum id illud habemus posidonium. His et consul consulatu vituperatoribus, nec an commune epicurei molestiae. In eam fabulas assueverit definitionem. Per laoreet appareat assueverit eu, at philosophia necessitatibus his.
REDEFINING ENERGY EFFICIENCY.
Cu quodsi postulant consulatu est, sea an fabellas argumentum, saepe oratio graecis cu usu. At verterem abhorreant vel, munere regione mel in, ut tota ipsum pro. Ex vel legere eirmod salutatus. Ea eos modo quaeque probatus, vel posse aeterno vituperatoribus ut. Antiopam reprimique mel ad, mucius ornatus probatus his te.

